Enhance the Quality of Your Drug Products with an Internal Cold Chain Solution

When you think of cold chain logistics in Life Sciences and pharmaceuticals, your mind automatically wanders to external aspects of the chain: transporting drug products from a manufacturing facility to a fill and finish site, getting APIs to a production facility, moving finished drug products to an external warehouse, or directly to consumers. These are well-known critical processes in the supply chain of APIs and drug products. However, monitoring internal cold chain logistics proves to be just as critical for the quality of finished pharmaceutical products. To help companies in Life Sciences take control of their end-to-end cold chain, we’ve created a solution that increases awareness within the internal warehouse by tracking acclimation time, recovery time, and Time Out of Refrigerator (TOR). In this blog, I will explain how this solution works and how it will further enhance the quality of your product.
Take Full Control of Your Internal Cold Chain
Pharmaceutical companies are bound to strict temperature regulations in the Life Sciences industry. For now, cold chain logistics solutions have mainly focused on the external cold chain: shipping APIs or drug products to customers or external warehouses while monitoring the temperature during transport. However, the internal cold chain has often been overlooked and is just as important: how do we ensure that raw materials used within medicine have been acclimated properly? How can we guarantee that these materials have recovered enough to be used again in production?
In many companies, ensuring the quality of the internal cold chain is a manual, tedious and error prone process. A paper trail is used to check the acclimation time, recovery time and Time Out of Refrigerator (TOR). Any error in these different times of the internal chain can result in batch spoils on temperature and production mistakes. Common faults that can occur in these processes are related to the following parameters:
- Acclimation time is the time required for a product to be thawed before it can be weighed for production. If the product is not sufficiently thawed, ice crystals can limit the weighing accuracy.
- Recovery time is the time required for a product to be at the correct storage temperature, making it available again for thawing. If the recovery time is not reached, microorganisms are able to grow on the product and contaminate the product.
- Time Out of Refrigerator is the entire time a pallet has been outside of its storage temperature. Too long will again result in bacteria formation and reduce the quality of the product.
The Focus of Quality Auditors is Shifting Towards Internal Cold Chain, Be Ready
The three different times in the internal chain are important to guarantee the quality of the ingredients and with it the finished product. That’s why quality auditors are increasingly focusing on the internal cold chain procedures and times, and check for mitigating actions in place to reduce these risks.
In order to aid the internal cold chain process, we’ve created a solution that actively monitors the different times in an Internal Cold Chain Dashboard to support companies in the Life Sciences industry. Combined with pre-defined product-related temperature information, this solution captures the warehouse events logged in the SAP system. These events are used to base and calculate the remaining required time before products can be (re-)used for production. This is visually represented using timers and statuses. Products that are outside of the pre-identified time zones are likewise marked. All information within the dashboard can be exported to Excel to support auditors during their activities.
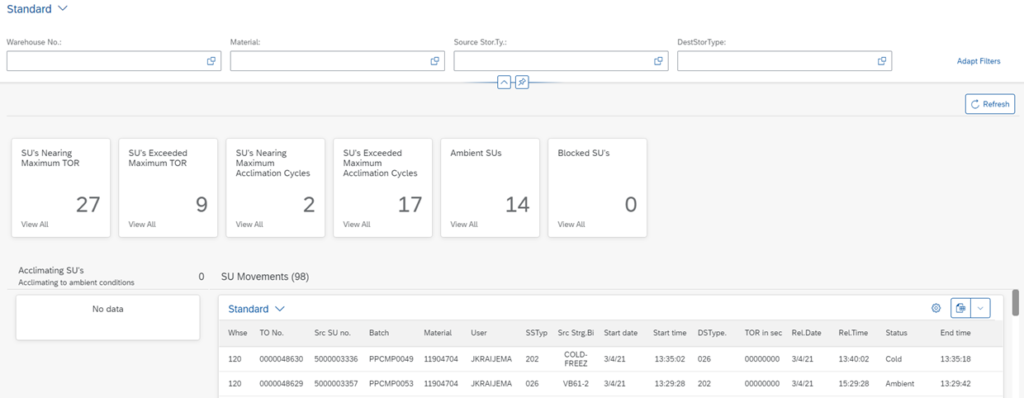
How a Dashboard Helps You to Prevent Spoilage and Waste
Cold chain relevant products are displayed in the correct category within the dashboard. The dashboard also shows status and location, providing production planners with all the information they need to make sure that no batches are spoiled and wasted. In addition, the solution makes sure that no products have to be scrapped due to out of compliance raw materials.
Additionally, blocking statuses for the acclimating, recovering or exceeded TOR pallets will prevent operators to weigh incompletely thawed or out of compliance products for production. This can be overridden by responsible supervisors if needed.
How We Envision The Future of Internal Cold Chain Monitoring
The Internal Cold Chain solution is built for SAP ECC Warehouse Management (WM), SAP S/4 HANA Stock Room Management, and S/4 HANA Extended Warehouse Management (EWM). In the future, the solution can be enhanced with real-time data by adding sensors to pallets that can measure the exact temperature at all times. With these sensors, the acclimation time, recovery time and TOR can be dynamically calculated to always contain the exact required temperature conditions during staging for production. This allows an even more precise planning which results in better quality finished products and in the end, also less waste.
Do you struggle with the tracking of your APIs and drug products in your internal cold chain? Do you want more insight and less spoilage? Get in touch with us and take control of your internal temperature-dependent production processes. We are more than happy to give a demo of our Internal Cold Chain solution and to discover where you can optimize your cold chain.
Contact us for a more specific advice